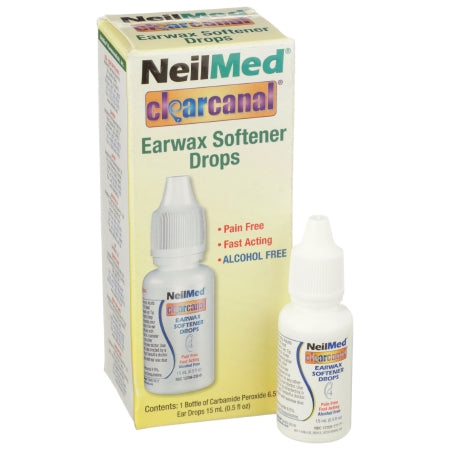
Description
Features
- Alcohol-free, non-irritating
Attributes
- Active Ingredients: Carbamide Peroxide
- Application: Ear Wax Remover
- Container Type: Dropper Bottle
- Dosage Form: Otic Drops
- FSA Eligible – Primary UOM: Yes
- FSA Eligible – Sell UOM: Yes
- Is_Active_Vendor: Y
- Is_DSCSA: N
- Is_Discontinued: N
- Is_Medical_Device: N
- Lot_Tracking_Flag: N
- NDC Number: 13709023001
- On_Allocation: N
- Product Dating: McKesson Acceptable Dating: we will ship >= 180 days
- Strength: 6.5% Strength
- Supplier_ID: 3884928
- UNSPSC Code: 51473503
- Volume: 0.5 oz.



Reviews
There are no reviews yet.