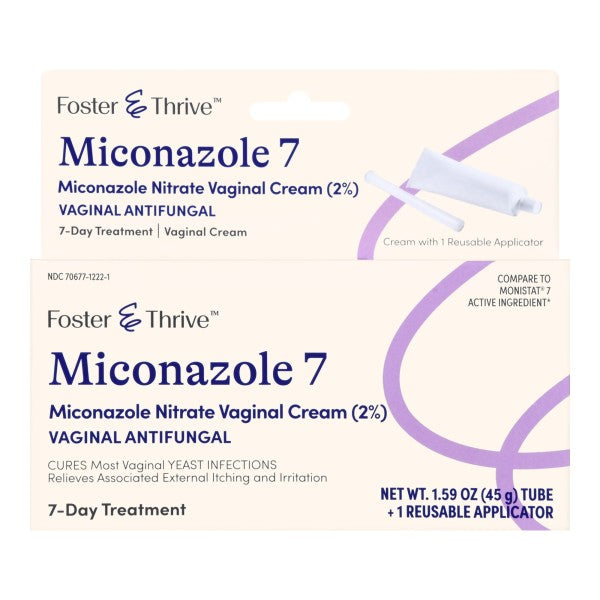
Description
Features
- Treats vaginal yeast infections
- 3 prefilled cream applicators
- Cures most vaginal yeast infections
Attributes
- Active Ingredients: Miconazole Nitrate
- Application: Vaginal Antifungal
- Container Type: Bottle
- Dosage Form: Suppository
- FSA Eligible – Buy UOM: Yes
- FSA Eligible – Primary UOM: Yes
- FSA Eligible – Sell UOM: Yes
- Is_Active_Vendor: Y
- Is_DSCSA: N
- Is_Discontinued: N
- Is_Medical_Device: N
- Lot_Tracking_Flag: N
- NDC Number: 63736001518
- On_Allocation: N
- Product Dating: McKesson Acceptable Dating: we will ship >= 180 days
- Strength: 4% Strength
- Supplier_ID: 3885243
- UNSPSC Code: 51302337
- Volume: 3 per Box





Reviews
There are no reviews yet.